Abstract
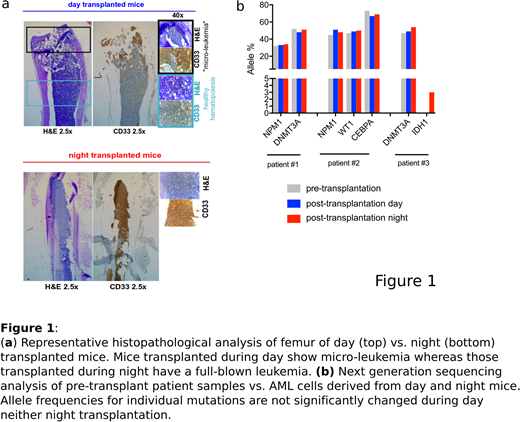
Mouse xenografts are routinely used for in vivo studies on human acute myeloid leukemia (AML) cells. However, a significant proportion of primary AML samples (~50-60% of cases) are not able to repopulate mice, or respectively require a particularly long time (up to 9 months) to show detectable engraftment in such models. Here we report that changing the transplantation time-point from day (5:00 pm) to night (4:00 am) strongly improved the engraftment of human AML cells in NOD/SCID/IL2Rgnull (NSG) mice. Sublethally irradiated gender-matched NSG mice of 6-8 weeks of age (n=40) were transplanted with primary AML cells (n=5 patients). For each sample, equal numbers of freshly thawed AML cells were injected via the tail vein at both time-points; transplanted mice were afterwards monitored for human leukemic engraftment in peripheral blood (PB) and bone marrow (BM) using multicolor flow cytometry and immunohistochemistry. In all n=5 analyzed AML patient samples, transplantation at night strongly promoted leukemogenesis, with 2/5 samples showing engraftment only in the night transplant condition and 3/5 samples showing engraftment in both but significantly higher leukemic burden in night vs. day transplanted mice (Fig. 1a), although similar leukemia-specific mutations were retrieved in mice of the two conditions by next generation sequencing analysis (Fig. 1b). Limiting dilution experiments (n=2 AML samples) further confirmed these findings and showed that in fact lower numbers of AML cells were required to initiate leukemia when cells were transplanted at night versus day times.
Given that the immediate process that happens after intravenous injection of AML cells into mice involves homing to BM niches, we hypothesized that the time-point of transplantation influences engraftment by modulating homing. Therefore, we performed homing assays as described for healthy hematopoietic stem/progenitor cells (HSPCs); we injected equally treated human CFSE labeled AML cells via tail vein injection at day versus night time points and analyzed murine BMs 8 hours later for the presence of human leukemic cells. Indeed, significantly higher BM colonization by leukemic cells was observed after night vs. day time injections (0.31±0.09 vs. 0.13±0.18, p<0.001, n= 7 AML patient samples).
Since mice transplanted at night showed stress-related behavioral abnormalities, we hypothesized that this transplantation method might induce catecholamine release. ELISAs indeed revealed enhanced epinephrine levels in PB (318.00±12.96 vs. 122.20±47.11 ng/mL, p<0.001) as well as BM (21.08±1.04 vs. 13.50±3.57 ng/mL, p<0.05) of night vs. day transplanted animals. The role of catecholamines influencing leukemia initiation is underinvestigated; b-adrenergic receptors are expressed on several cell types in the BM including HSCs, osteoblasts and mesenchymal stem cells and catecholamine release was shown to regulate HSC egress from BM niches. We hypothesized that enhanced adrenergic signaling may promote leukemogenesis by enhancing leukemic cell homing to BM niches, which in this setting are more readily available due to enhanced healthy HSC egress. To explore this hypothesis, we treated mice with the b1/2-blocker propranolol prior to night transplantation (10mg/kg, intraperitoneal (i.p) injection, 1h pre-transplant, to reverse the "night effect") and respectively with epinephrine prior to day transplantation (2mg/kg, i.p injection, 1h pre-transplant, to mimic the "night effect"). Indeed, propranolol treatment reduced homing and delayed leukemogenesis in night transplanted mice. Vice-versa, epinephrine enhanced AML cell homing and accelerated long-term leukemia induction from patient derived AML cells and cells of the EOL-1 cell line, and these effects could be counteracted by co-treatment with propranolol. Interestingly, in vitro co-cultures with epinephrine versus empty carrier control enhanced AML cell but not healthy HSPC adhesion to MS5 stroma cells (shown for n=3 cell lines and n=8 primary AML), suggesting that enhanced catecholamine levels may specifically induce AML cell adhesion.
We conclude that enhanced catecholamine levels can accelerate leukemia development from AML cells transplanted in xenograft models by facilitating BM niche colonization by AML cells. Catecholamines might in general promote leukemia progression by favoring the retention of leukemic versus healthy HSCs in BM niches.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal